

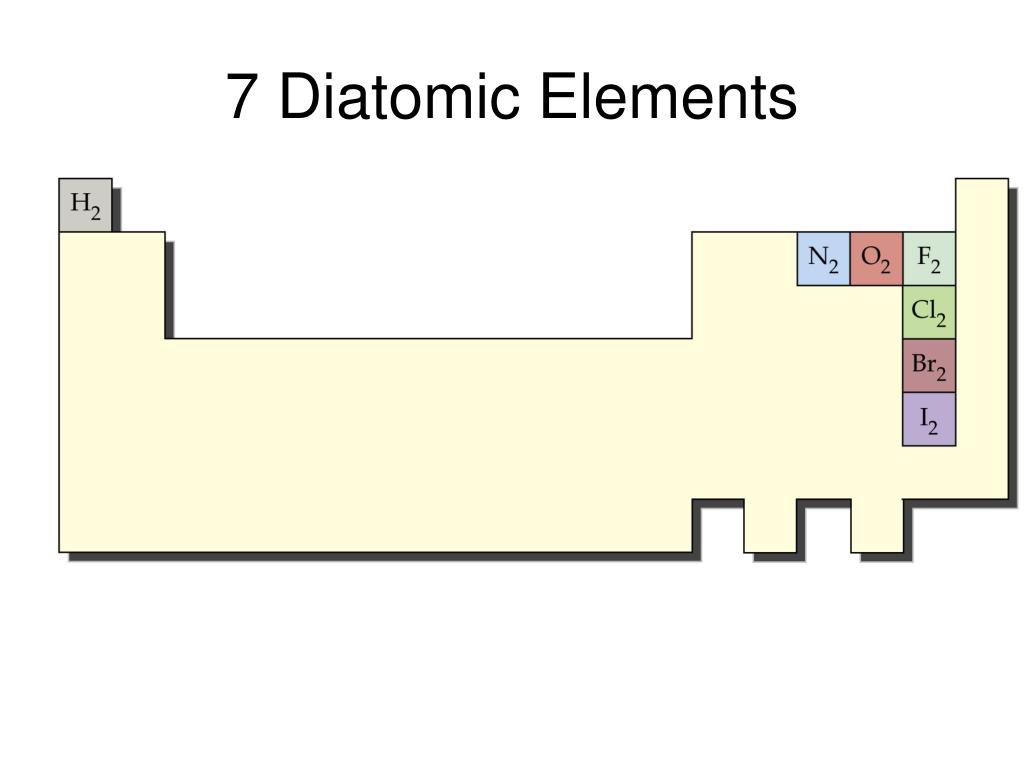
When there is a single p-orbital containing a pair of electrons, this pairing results in increasing the energy of the orbital. S-p mixing occurs only when the s and p- orbitals possess similar energies. Similar effect is observed on antibonding orbitals and thus, σs* becomes more stable and σp* becomes less stable. σs combines with σp which results in stabilizing σs orbitals and σp orbital become less stable. This mixing influences the energies of the molecular orbitals. This decrease in orbital energy changes the bond order in orbitals of N₂ and O₂ which occurs due to s-p mixing. The 1σs bonding orbital is shown below:Īcross the period, the effective nuclear charge increases, which leads to decreasing the atomic radius and thus, result in a decrease in orbital energies. Further, the electrons occupy the molecular orbital possessing the lowest energy. The combination of atomic orbitals of two atoms results in the formation of the molecular orbital. Two hydrogen atoms combine to form a hydrogen molecule. This theory is helpful in reasoning the existence of molecules, prediction of bond strength, bond order, magnetic properties, and electronic transitions that can take place. According to these theories, molecular orbitals are formed by combining atomic orbitals that possess comparatively the same energy. Molecular orbital diagram, which is also known as MO diagram helps in explaining the chemical bonding present in molecules in terms of LCAO, a linear combination of atomic orbital method and molecular orbital theory in general. If the electronegativity difference between the elements is in the range of 0.5 to 1.9, then the bond formed is a polar covalent bond like CO, whereas the complete transfer of electron results in an ionic compound like NaCl. For example, nitric oxide (NO), hydrochloric acid (HCl), and carbon monoxide (CO). Many elements can combine to form heteronuclear diatomic molecules depending on temperature and pressure. The molecule composed of two atoms of different elements is known as heteronuclear diatomic molecules. Thus, the bond present in them is a non-polar covalent bond. The electronegativity difference is zero for the homonuclear diatomic molecules. Five of these elements that occur as diatomic elements at room temperature (25 ⁰ C) are Hydrogen (H₂ ), fluorine (F₂ ), nitrogen (N₂ ), chlorine (Cl₂ ), and oxygen (O₂ ).Īt higher temperature, bromine (Br₂ ) and iodine (I₂ ) exist as homonuclear diatomic molecules. Naturally occurring homonuclear diatomic molecules are known for seven elements that are present in a gaseous state.

The molecule composed of two atoms of the same element is named as homonuclear diatomic molecules. They are homonuclear and heteronuclear diatomic molecules. The two types of diatomic molecules are determined on the basis of the type of elements that are involved in the molecule. These ions are attracted to each other via electrostatic force of attraction and therefore the ionic bond is strong in nature. Thus, it results in the formation of ions, namely, a cation and an anion, respectively. In this type of bond, a metal loses an electron(s) and non-metals gain an electron(s). The electronegativity difference between the bonded atoms should be less than 0.5.Ĭomplete transfer of electrons between the atoms results in the formation of an ionic bond. Thus, the pair of electrons between two atoms are shared equally. This bond is basically formed when electronegativity values of two atoms are the same. The range of electronegativity difference between the bonded atoms should be in 0.5-1.9. Thus, the pair of electrons between two atoms are shared unequally. This bond is formed due to the difference in the electronegativity values of two atoms. They are polar and non-polar covalent bonds. Atoms can form one to three covalent bonds depending on the number of valence electrons involved in the bond formation.Ĭovalent bonds are also sub-divided into two types.

These bonds are formed by sharing the valence electrons (outermost shell electrons) of the atoms.
The sharing of pairs of electrons between the atoms results in the formation of a chemical bond, and this type of bond is known as a covalent bond.


 0 kommentar(er)
0 kommentar(er)
